Avoid a Compliance Nightmare This Halloween
As Halloween approaches, it’s the perfect time to revisit some of the most frightening cleanroom compliance missteps we’ve seen in the field. At Contec Healthcare, we’ve spent decades working with compounding pharmacies to prevent contamination, improve USP <797> and <800> compliance, and support better patient outcomes. In our Contec-hosted webinar, our team shared real-world examples of critical pharmacy fails—each one an opportunity to learn and improve.
Here’s a closer look at the scariest (and surprisingly common) mistakes we’ve encountered—and what your team can do to stay safe, compliant, and audit-ready all year long.
Inadequate Gowning: A Gateway to Contamination
One of the most common and concerning fails our team sees is the use of improper PPE, especially gowns and shoe covers. Many pharmacies rely on general-use spun bound polypropylene gowns that don’t offer proper particle control or barrier protection. These garments shed fibers, provide minimal resistance to microbial penetration, and can compromise your cleanroom’s ISO classification.
Best Practice: Use cleanroom-appropriate garments with microporous laminate materials, mandarin collars, thumb loops, and full coverage from neck to knees. Shoe covers should also be low-linting and durable enough to withstand friction from cleanroom flooring. Avoid blue polypropylene booties—the kind that leave visible fuzz balls and particle trails on mops and floors. Contec offers PPE solutions designed specifically for sterile compounding environments.
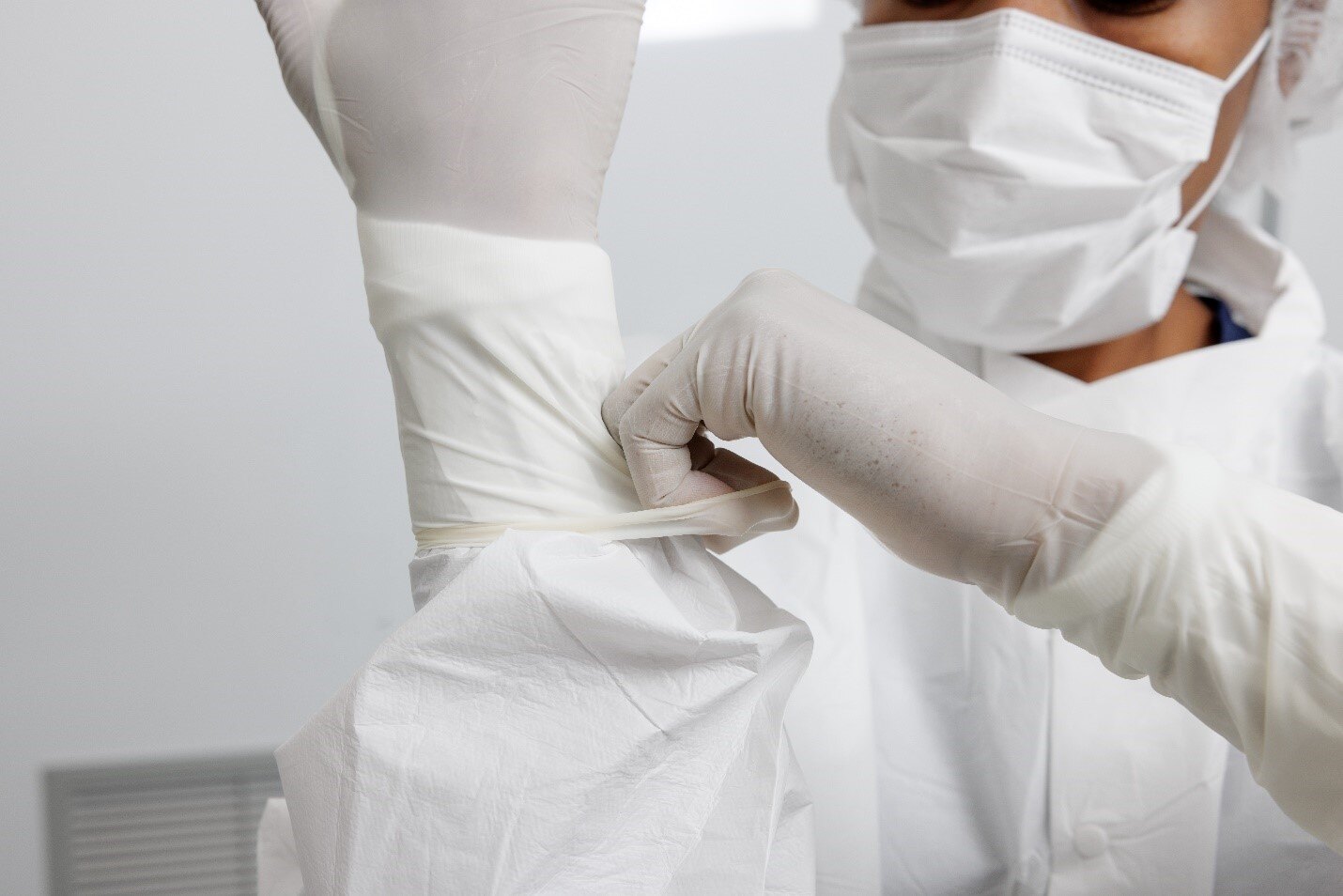
Improper Glove Selection: A Silent Compliance Violation
Another common mistake is the use of sterile surgical gloves in place of sterile cleanroom gloves. While both may be sterile, surgical gloves often come in fibrous-paper inner packaging that sheds particles. The gloves themselves are not processed in a cleanroom environment and may introduce unwanted contamination during donning.
Best Practice: Use sterile cleanroom gloves that have been laundered in a cleanroom laundry, packaged in plastic inner wrappings, and designed for compatibility with cleanroom gowning procedures. Look for longer cuffs to ensure a secure interface with gown sleeves, and avoid “DIY” workarounds like cutting slits in cuffs to hold gloves in place—use thumb loops instead.
Tacky Mat Mistakes: Small Oversights, Big Consequences
While tacky mats are not a best-practice they are commonly used in compounding pharmacies. But these are often misused in cleanrooms, diminishing their effectiveness. The most common mistake? Installing them like a welcome mat—horizontal across the doorway—so that personnel only get a single step on the mat. Others forget to replace the sheets regularly, turning the mat into a source of contamination rather than a solution.
Best Practice: Tacky mats should be installed lengthwise across the walking path so that users take at least two to three steps across the adhesive surface. Establish a written protocol for how often sheets should be changed—ideally multiple times per day depending on traffic. Additionally, make sure the peel direction is away from cleanroom doors to prevent airborne particle release.
Cleaning Equipment from “Outside”: A Direct Violation
Facilities that rely on EVS (Environmental Services) or janitorial staff may unintentionally bring in non-compliant cleaning tools like string mops, wax buffers, or microfiber pads with Velcro—none of which are suitable for compounding pharmacies. In one alarming case, a cleaning crew used paint rollers secured with rubber bands to clean ISO-classified walls.
Best Practice: Use dedicated cleanroom cleaning tools stored within the pharmacy. All mop hardware and textiles should be validated for cleanroom use and stored properly to avoid cross-contamination. If your EVS team is involved in cleanroom maintenance, make sure they’re trained specifically in USP <797>/<800> cleaning protocols—or work with Contec to provide competency training and certification.
Breaking the Plane of the PEC: A Critical Violation
Some pharmacy staff use manual hand wipes to clean the back wall of a Primary Engineering Control (PEC), leaning their torso inside the cabinet in the process. This introduces exposed skin, breaks airflow patterns, and jeopardizes the sterility of the environment. The revised USP Chapter 797 specifically states that there must be no exposed skin inside the PEC.
Best Practice: Use tools like Contec’s EasyReach system to clean inside PECs without physically entering the space. The right cleaning tools allow staff to maintain unidirectional airflow, prevent recontamination, and eliminate the need to breach critical work zones.
Overusing or Misusing Disinfectants
Spraying disinfectants directly onto surfaces without control or using diluted, non-sterile disinfectants can result in inconsistent dwell times, residue buildup, and ineffective microbial kill. Some facilities even rely on hospital-wide disinfectants mixed with tap water—completely inappropriate for cleanroom use.
Best Practice: Use ready-to-use (RTU), sterile disinfectants with validated short dwell times—ideally 1 minute for daily use and 3 minutes for sporicidal. Avoid manual dilution, which introduces human error and compatibility risks. Contec offers sterile RTU disinfectants that eliminate the need for in-house dilution, support faster workflow, and help meet evolving USP expectations.
Poor Material Compatibility: Damaging Surfaces & Equipment
One hospital blamed a disinfectant for corroding their compounding workbench—until it was revealed that the surface wasn’t cleanroom-grade stainless steel. Using the wrong materials in a cleanroom not only causes physical degradation, but also creates harbor points for bacteria.
Best Practice: Ensure all surfaces are smooth, impervious, non-shedding, and easy to clean, per USP <797> (2008 and upcoming revisions). Use 304 or 316-grade stainless steel, compatible flooring, and cleanroom-validated furniture throughout the pharmacy.
Lack of Documentation & SOP Alignment
Even when facilities have the right products in place, failure to document cleaning procedures, expiration dates, PPE storage, or disinfectant rotation can lead to major audit findings.
Best Practice: Maintain clear, up-to-date SOPs aligned with USP <797>/<800>, and ensure all staff are trained and competent. Don’t just write it—inspect it. Verify that practices match documentation and that logs are completed consistently.
In Conclusion
A cleanroom doesn’t stay clean on its own—it requires a system of intentional decisions, compliant products, and trained personnel. Every oversight—from gowning to glove choice, from mop type to disinfectant dwell time—creates risk. But with the right partners and protocols in place, those scary compliance nightmares can be put to rest.
Contec Healthcare is here to help. From selecting the right cleanroom supplies to training your team on best practices, we support your pharmacy’s goal to deliver safe, compliant, high-quality compounded medications.
Don’t wait for a compliance scare! Contact Contec Expert to schedule a cleanroom evaluation or product consultation today.

-medium.jpg)