The Role of USA-Made Cleaning Supplies in Compounding Pharmacy Operations
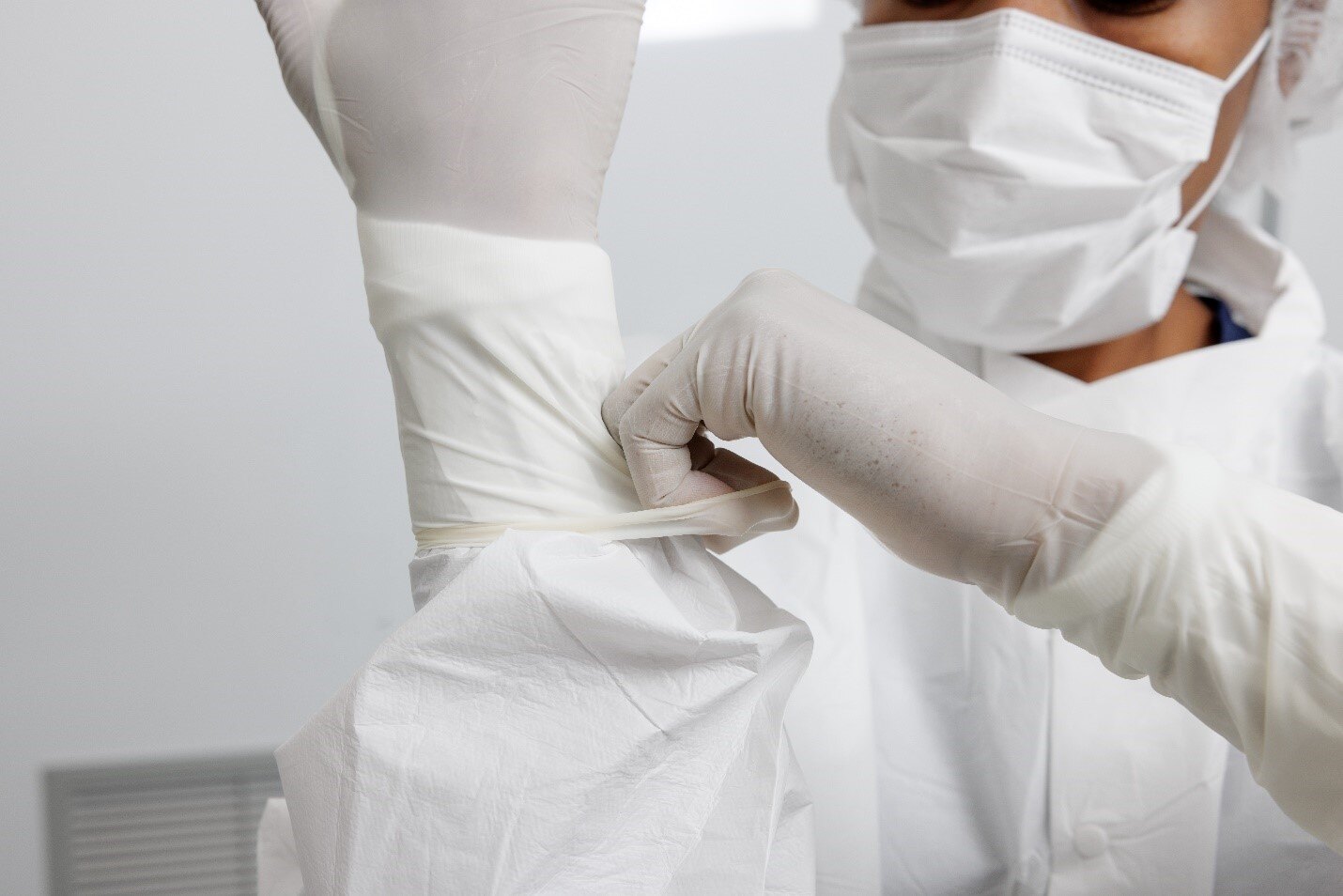
For compounding pharmacies, maintaining a sterile and controlled environment is non-negotiable when it comes to patient safety. Daily cleaning and disinfection routines must be supported by tools that are consistent, validated, and compatible with USP standards. As a supplier of USA-manufactured wipes, mops, and disinfectants, we’ve seen firsthand how domestically made products help facilities meet both operational and regulatory demands with greater reliability.
Quality That Supports Cleanroom Standards
Products used in cleanrooms must meet specific material and performance criteria—especially in ISO Class 5 environments, where sterile compounding is performed. USA-made wipes and mops are manufactured under stringent quality control systems, in ISO-certified or GMP-compliant facilities. This translates to better consistency in key areas such as particle control, absorbency, and compatibility with commonly used disinfectants.
Sterile wipes are typically produced using low-linting and are sterilized using validated methods like gamma irradiation or ethylene oxide. These products are also packaged specifically for cleanroom workflows, often double- or triple-bagged to support aseptic transfer. Domestic manufacturers tend to follow transparent and rigorous quality systems, making it easier for pharmacies to trust the performance of the products they use to maintain sterile environments.
Supply Chain Stability and Accessibility
Access to a consistent, on-time supply of cleaning materials is critical for pharmacies operating under USP <797> and <800> requirements. Daily disinfection of work surfaces, weekly sporicidal cleaning, and routine floor mopping all require dependable access to sterile and nonsterile products. Delays or stockouts can compromise environmental control, forcing deviations from established procedures.
Domestic sourcing minimizes many of the risks associated with international shipping, customs delays, and unpredictable availability. Pharmacies relying on USA-made products often experience fewer disruptions, which makes it easier to maintain cleaning schedules and meet operational targets. Additionally, domestic suppliers can often provide a quicker turnaround on technical questions, documentation requests, or support when a product-related issue arises.
Alignment with Regulatory Requirements
Regulatory expectations under USP <797> and <800> continue to evolve, and cleaning products must keep pace with these changes. USA-made disinfectants and wipes are frequently developed with these standards in mind, including validated claims against bacteria, fungi, and bacterial spores to support disinfectant protocols. Many also include documentation such as Certificates of Analysis, sterility data, and compatibility information—resources that inspectors may request during audits.
For pharmacies handling hazardous drugs, material compatibility is critical. Products need to withstand repeated exposure to decontamination agents without degradation. This level of compatibility is easier to confirm when sourcing from manufacturers who provide transparent material specifications and testing data.
At Contec® we have made it our focus to supply compounding pharmacies with USA-made cleaning solutions that meet the demands of cleanroom environments. Whether you need sterile wipes, EPA-registered disinfectants, or mop systems compatible with USP <797> and <800> workflows, our team works to ensure you have access to the documentation, performance, and support you need to maintain compliance with confidence.



-medium.jpg)
