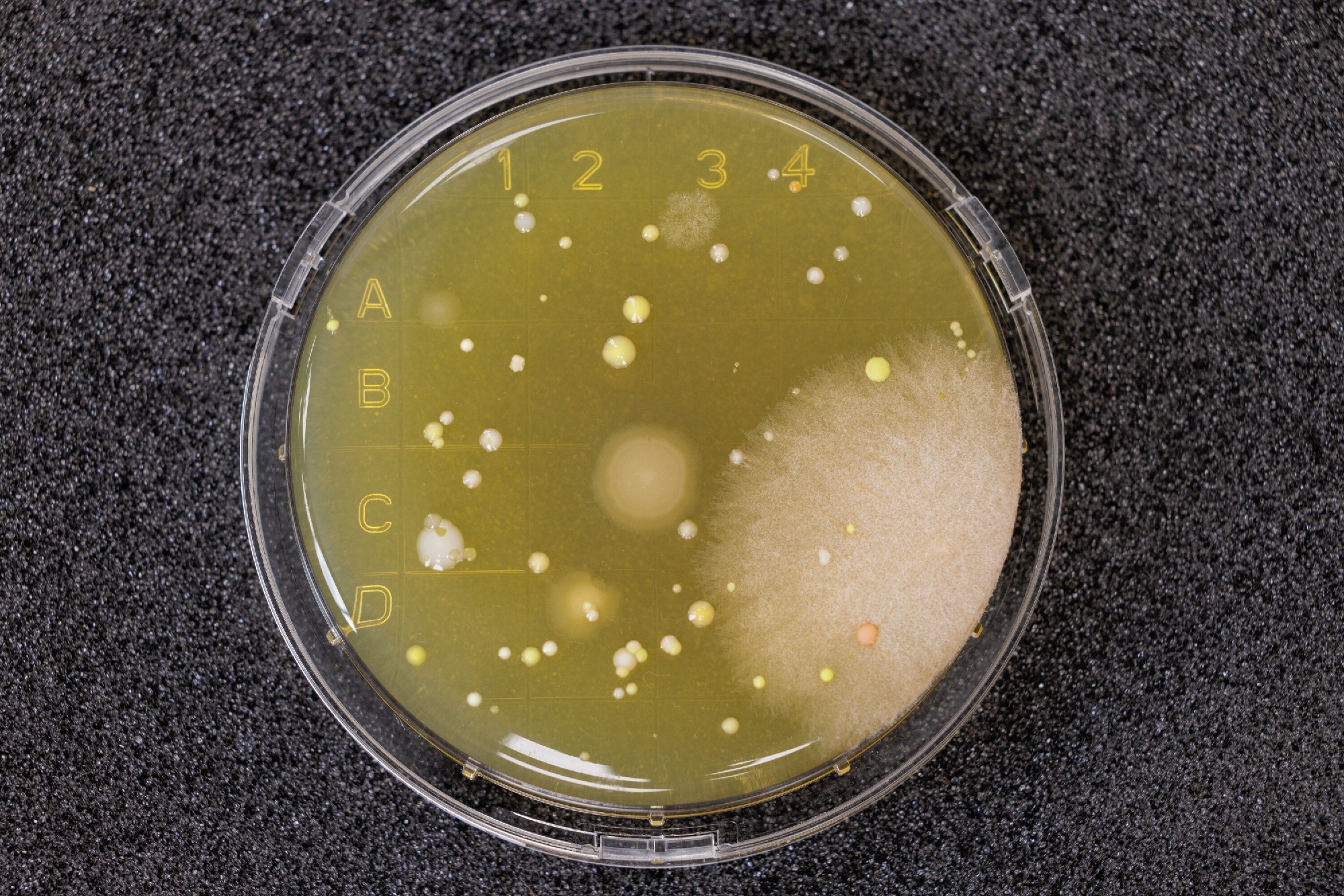
The only mechanism to assess microbial contamination in the sterile compounding environment is viable sampling. However, dealing with microorganisms is like dealing with toddlers, we never know how they are going to behave.
Many of you have started collecting viable surface samples monthly, or even weekly if you compound Category 3 preparations. This increased sampling frequency will likely result in more excursions and microbial investigations.
As you potentially face more excursions, there are a few viable sampling challenges and limitations you need to keep in mind. Understanding these challenges and limitations will help keep you grounded as you tackle your investigations.
Challenge #1: Microbial contamination is inevitable and expected!
People prepare sterile preparations, and we harbor microorganisms. We can’t eliminate people, so we need to mitigate risk through garbing, behavior, material movement, and cleaning. Remember, engineering controls cannot prevent contamination, they can only remove the contamination brought into the classified space.
Challenge #2: The cleanroom suite and primary engineering controls are not sterile!
And if the cleanroom suite is not sterile, the segregated compounding area (SCA) is absolutely not sterile. EVER! Even after cleaning the sterile compounding areas, under static conditions, there is a chance microorganisms are present. The goal for all the compounding areas is LOW contamination.
The following viable sampling limitations are adapted from:
USP <1116> Microbiological Control and Monitoring of Aseptic Processing Environments.

Limitation #1: Compounders should review environmental monitoring results frequently to ensure a state of control, but monitoring can neither prove nor disprove sterility. Viable sampling must not be used solely as a release test for compounded sterile preparations.
Limitation #2: Viable sampling cannot and need not identify and quantify all microorganisms in the sterile compounding area. It is semiquantitative. This significant limitation exists because of the growth-based methods used in sample collection. We will only recover the microorganisms that like the media, incubation time, and incubation temperatures we provide. However, we need to remember that viable sampling is a spot check, or a snapshot of what was happening during collection. If there are consistently exceeded levels, it indicates a loss of microbial control.
Limitation #3: The sampling plan cannot prove absence of contamination, even when no growth was recovered. Microorganisms could still have been present during sampling. You just didn’t recover them. If you consistently get zeros during DYNAMIC operating conditions, it is going to be a red flag to inspectors. They will want to know why you haven’t recovered anything, as we expect to recover some microorganisms during operations. Be sure you aren’t cleaning before sampling and that your media will support growth.
Remember, viable sampling is not perfect. It comes with its challenges and limitations but is a great tool for process improvement.
If there are microorganisms present in the environment, we want to find them, remediate, and improve our procedures. By keeping this mindset, you will be sure to create and maintain a successful viable sampling program.